Benefits of the Data Model
The MDKU data model will be published free of charge in order to support the industry.
Standardize language
Through the unambiguous description of regulatory terms, intended for creation of clarity as to what is understood by a term or is expected by notified bodies. Today, terms are sometimes defined differently in several regulatory documents, interpreted incorrectly, or used in slightly different ways.
Accelerate digitization
through a uniform data model that can be used by all stakeholders in the MedTech ecosystem and implemented by all software providers. This also enables consistency and a digital thread between manufacturers, notified bodies, suppliers and other parties.
Goals: efficient creation, review and management of Technical Documentation
With the unified data model, we can achieve many benefits for manufacturers as well as for Notified Bodies.
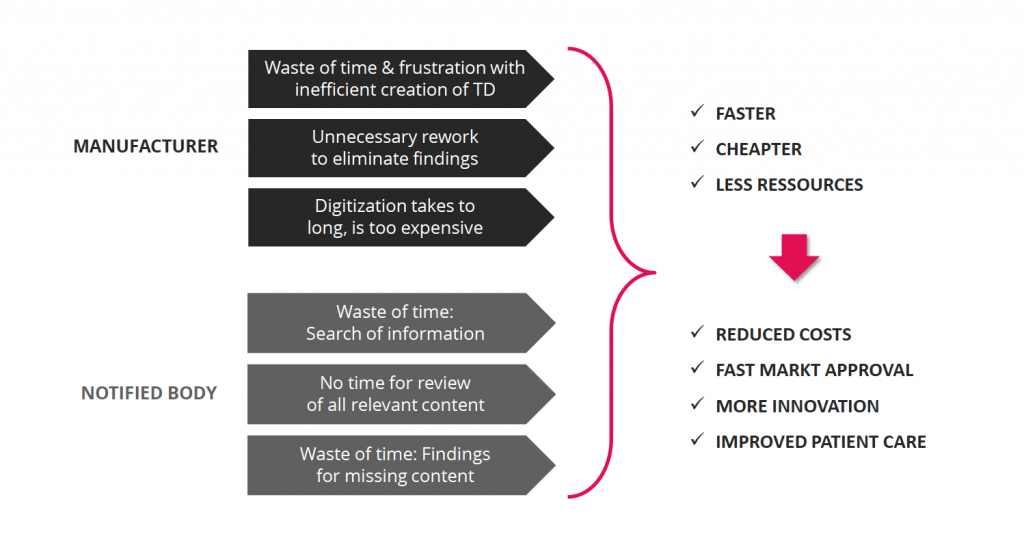
Impact of the Data Model
With a unified data model for content of the technical documentation we can easily achieve two main advantages:
1) A clear differentiation between the source of an information unit and the documents in which the information is reused
2) Automated completeness checks that ensure a more efficient review process and faster market approval.
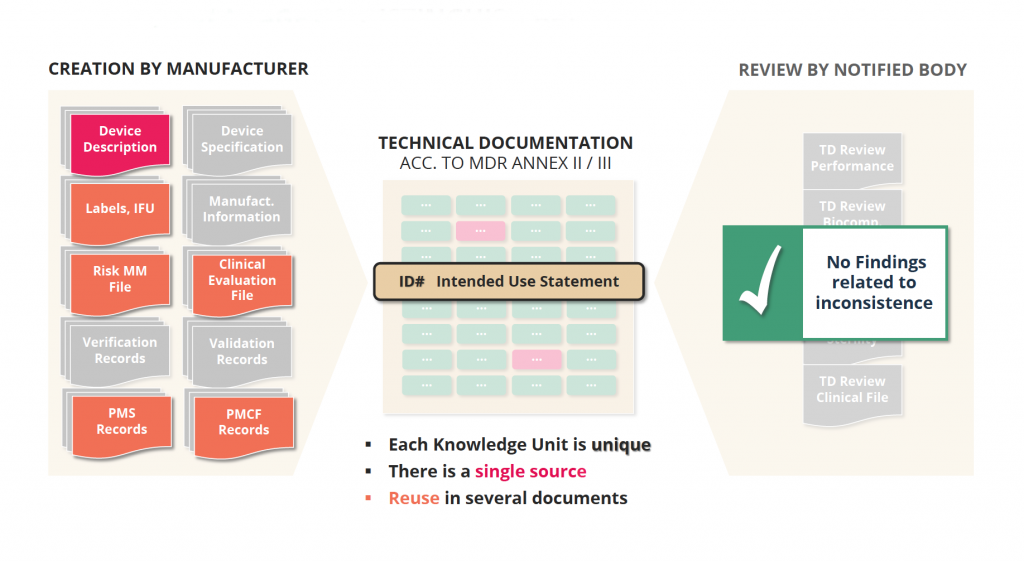
Advantage 1: Distinguish between Source / Reuse
Keep in mind: More than 50% of information in the technical documentation is used at least in two different documents, and the longer a product is on the market and the more post-market surveillance data will collected and design changes implemented, the higher the amount of reused information!
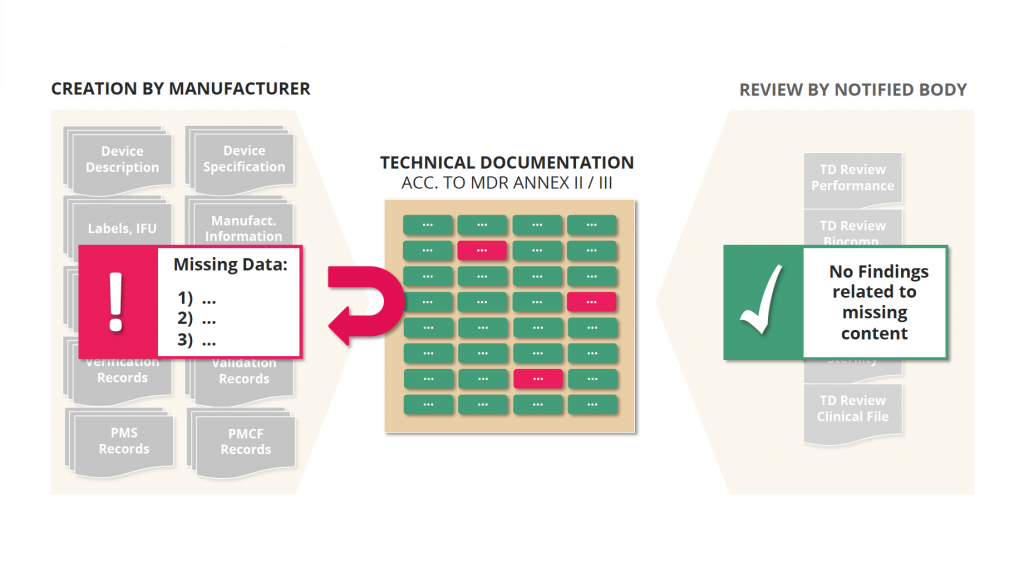
Advantage 2: Completeness Check
MDKU Medical Device Knowledge Units e.V.
Am Weichselgarten 7
91058 Erlangen
Germany