Situation in the MedTech Industry
The medical technology sector is one of the most innovative industries in the world, but the innovative strength of manufacturers is constantly being slowed down by ever-increasing regulatory requirements. Proof of the regulatory compliance of a medical device is provided on the basis of the technical documentation, which is the result of the product development process and various interface processes. If regulatory requirements are not fulfilled, manufacturers cannot sell products and therefore cannot operate successfully in economic terms.
Development of innovative diagnostic or therapeutic devices
Devices that reach market access in Europe
Surveys by industry associations show the problem: The innovation climate in medical technology is at a low point, and innovative devices are now first launched by many companies in other countries such as the USA or Japan.
The significance and impact of regulatory requirements is evident, among others, in the ever-increasing amount of documentation that has to be created in many corporate departments:
- Capacities of highly qualified employees are tied up and time for the activities that actually add value is reduced. The development of new, innovative diagnostic or therapeutic devices is significantly slowed down.
In Europe, due to extensive changes resulting from the MDR and IVDR, a new market approval is required for all medical devices and IVD devices already on the market, which means a revision of the technical documentation for each device. Capacity bottlenecks and a lack of specialists at manufacturers and Notified Bodies are currently causing a long delay in the re-registration process. As a result, resources for the development and approval of innovative medical devices in Europe have fallen sharply. This trend is expected to continue until at least 2028 (extended transition periods for the MDR/IVDR).
- This threatens the innovative capacity of the European medical device industry and, ultimately, the quality of patient care.
The MedTech "PAIN"
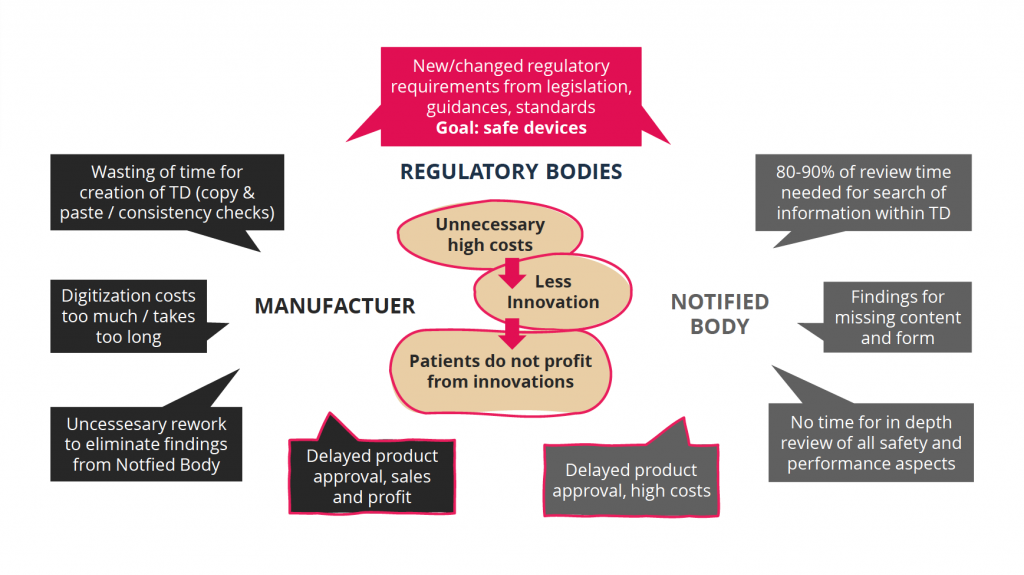
Regulatory requirements from laws, guidelines and standards aim to bring safer products to the market. However, the current situation in Europe is putting patient care at risk due to limited resources at medical device manufacturers and Notified Bodies.
How can we enable more innovation?
The digitalization of regulatory processes and the resulting digitalized technical documentation offers a great opportunity, which would eliminate typical current problems for manufacturers and significantly increase efficiency in the MedTech industry.
MDKU Medical Device Knowledge Units e.V.
Am Weichselgarten 7
91058 Erlangen
Germany