MedTech challenges that we address
When creating and maintaining technical documentation (TD), there are four challenges that have a major impact on the necessary effort:
Interpretation
We have hundreds of applicable regulatory documents in Europe from different sources, …
Consistency
Due to regulatory requirements, certain information is required in different places or documents …
Traceability
Single information elements within the TD are not only valid on their own, but are …
Completeness
Annex II and III of the MDR/IVDR describe the required contents of the TD. In practice …
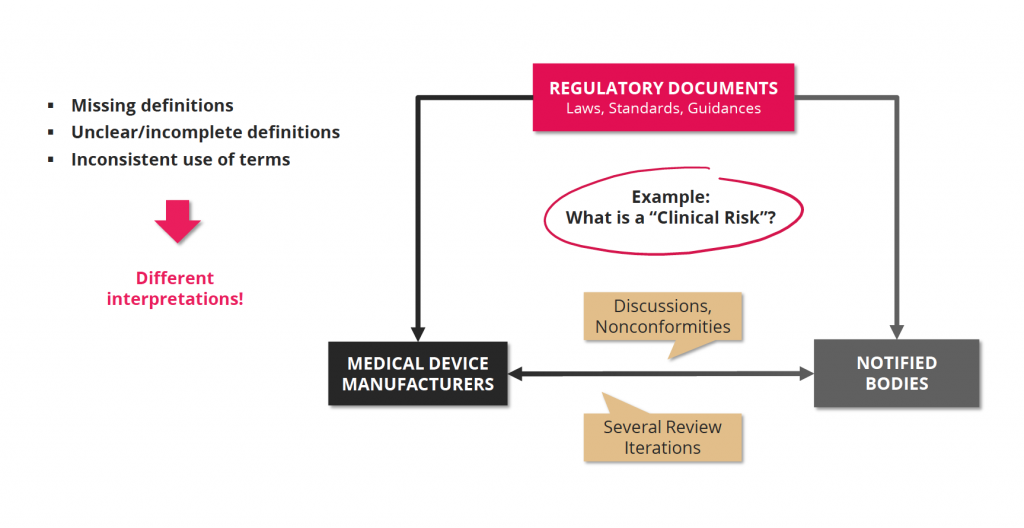
Interpretation of regulatory documents
We have hundreds of applicable regulatory documents in Europe from different sources, written by different authoring groups with different professional background. The terms used in those documents are often unclear and difficult to interpret. For some terms, definitions exists, for others not. The existing definitions are not always clear and „complete“ in a way, that the use of the term is clear for the reader. And, one of the biggest challenges that we see across many documents: terms are not used consistently by different authors.
The result? Endless hours of reading, comparison of documents, company internal discussions, discussions with notified bodies and so on. Time that is wasted and cannot be used for the development of new medical devices.
Challenge 1: Interpretation of regulatory documents
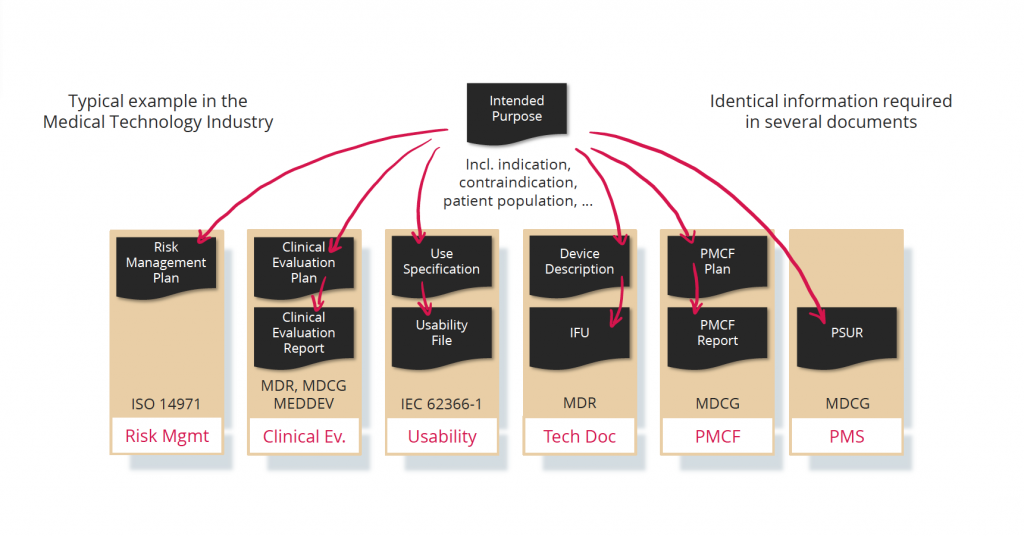
Reuse and consistency of information
Due to regulatory requirements, certain information is required in different places or documents within the TD (see example for the intended purpose). Of course, Notified Bodies expect that this information is consistent in all documents and kept up to date.
Challenge 2: Reuse and consistency of information
If the TD is created with Word or Excel, this means a large manual effort. The high complexity of information often leads to inconsistencies, which are typically identified during the review of the TD by Notified Bodies – leading to several review iterations and delayed market approval. According to expert estimates, on average about 50% of all information in a document is reused in at least one other document, often in several other documents (1). Furthermore, there are more and more regulatory required documents, which have led e.g. between 2017 and 2023 to an increase of about 600% of single documents only in the areas of Clinical Evaluation and Post-market Surveillance (2).
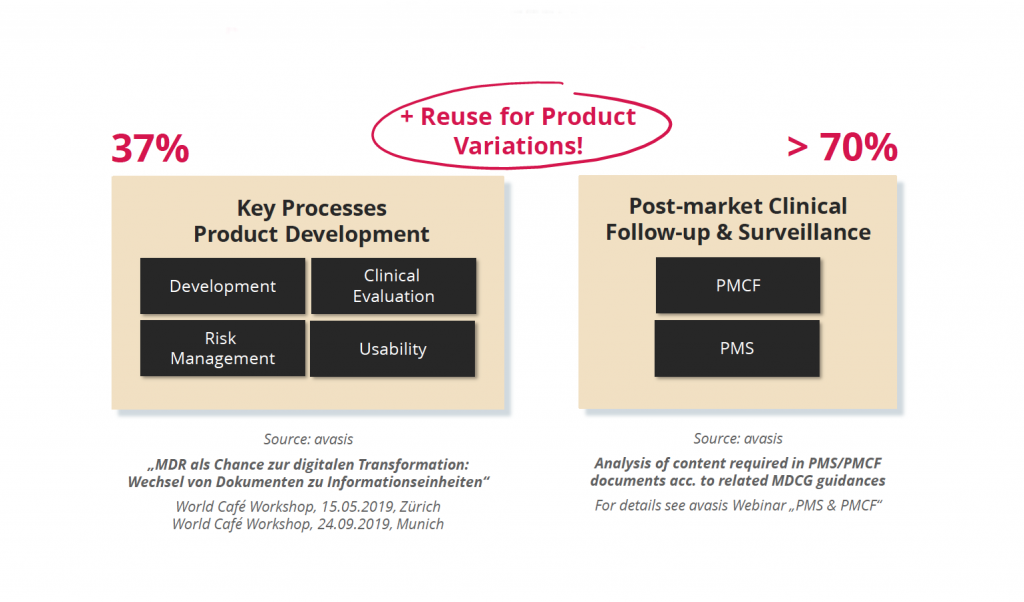
Results from workshops conducted by MedTech digitalization experts from avasis in 2019/2020
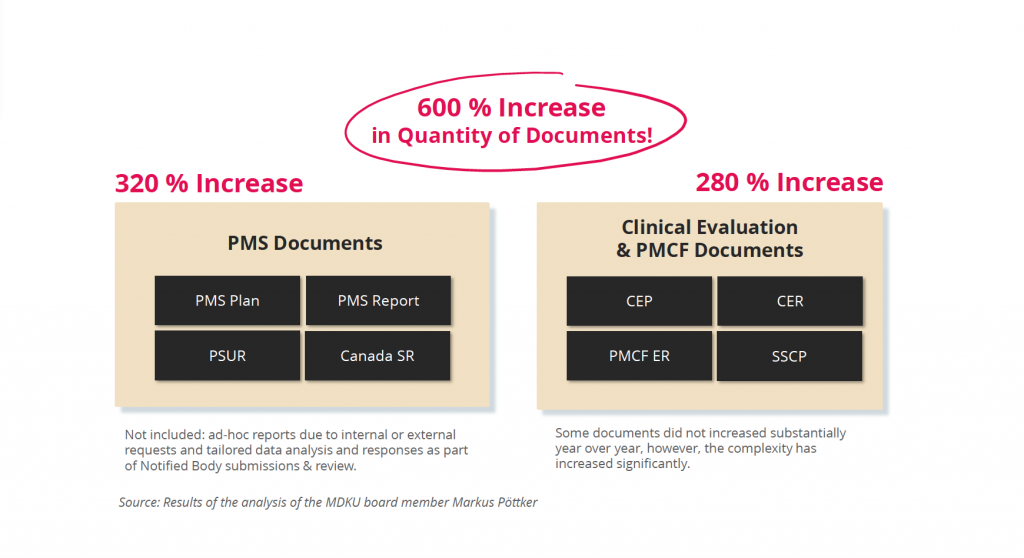
Additional challenge: amount of documents 2017 vs 2023
Dependency/traceability of information in different processes and documents
Single information elements within the TD are not only valid on their own, but are linked to other information or they have dependencies. Such links must be visible for an understanding of TD content, during creation and also review processes.
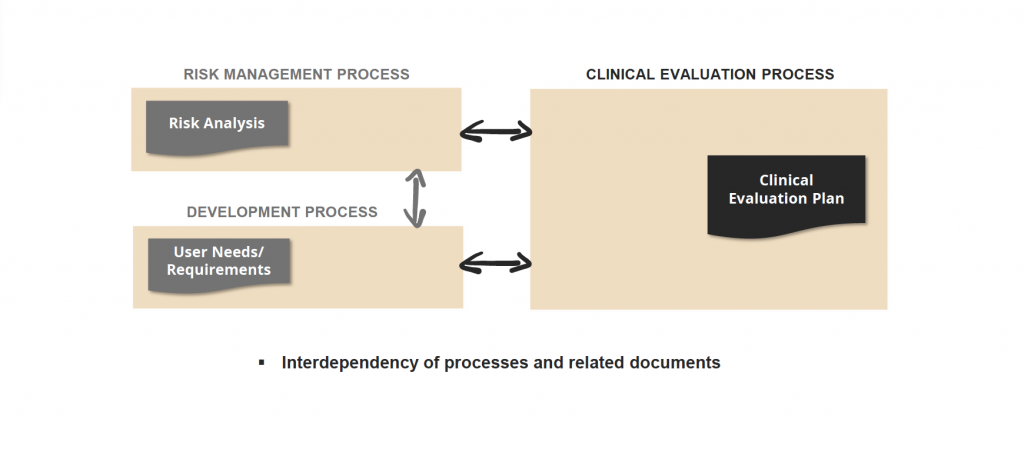
Challenge 3: complexity & traceabiltiy
For example, a requirement for the design of a product must be linked to a test description for demonstration of verification or validation activities. At the end, a valid test result is needed as evidence for regulatory compliance. Another example: the results of the usability engineering process must be used as input for the risk management process.
However, for the visualization of a traceability there must be a unique mapping of two information units. If the TD is based on Word/Excel, individual information units are usually numbered manually and the link is displayed in the form of a table. This means a high manual effort, including susceptibility to errors.
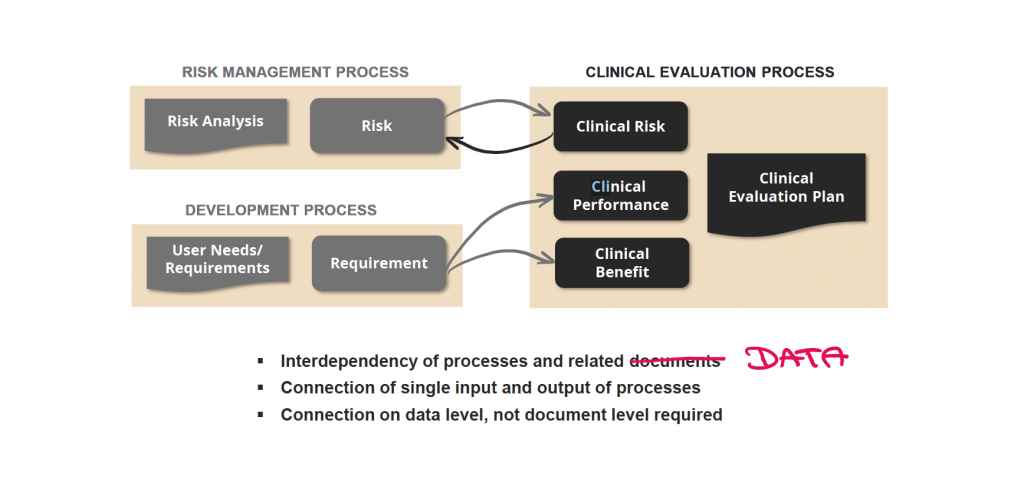
Interdepency of processes and related data
Completeness of required content
Annex II and III of the MDR/IVDR describe the required contents of the TD. In practice, a TD file consists of many individual documents, often hundreds, sometimes even thousands of pages long. The contents are created by different departments over a longer period of time. Due to the high complexity of processes and the created information, it is difficult for regulatory affairs managers to ensure that all regulatory required contents are complete when the TD is handed over to the Notified Body for review. According to statements made by Notified Bodies at various industry conferences, incomplete content is an issue for almost all TDs and massively delays the approval process of new devices.
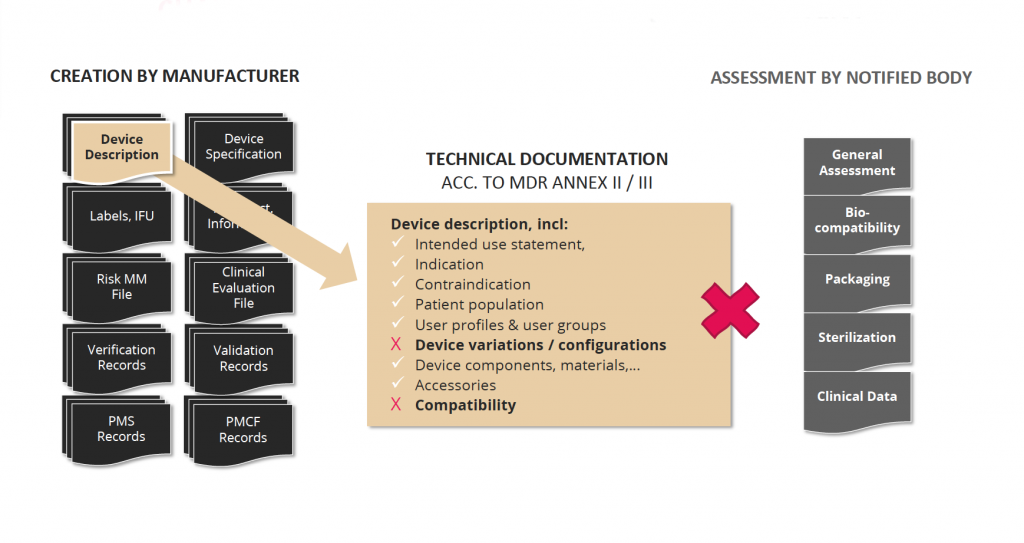
Challenge 4: completeness of required content
MDKU Medical Device Knowledge Units e.V.
Am Weichselgarten 7
91058 Erlangen
Germany